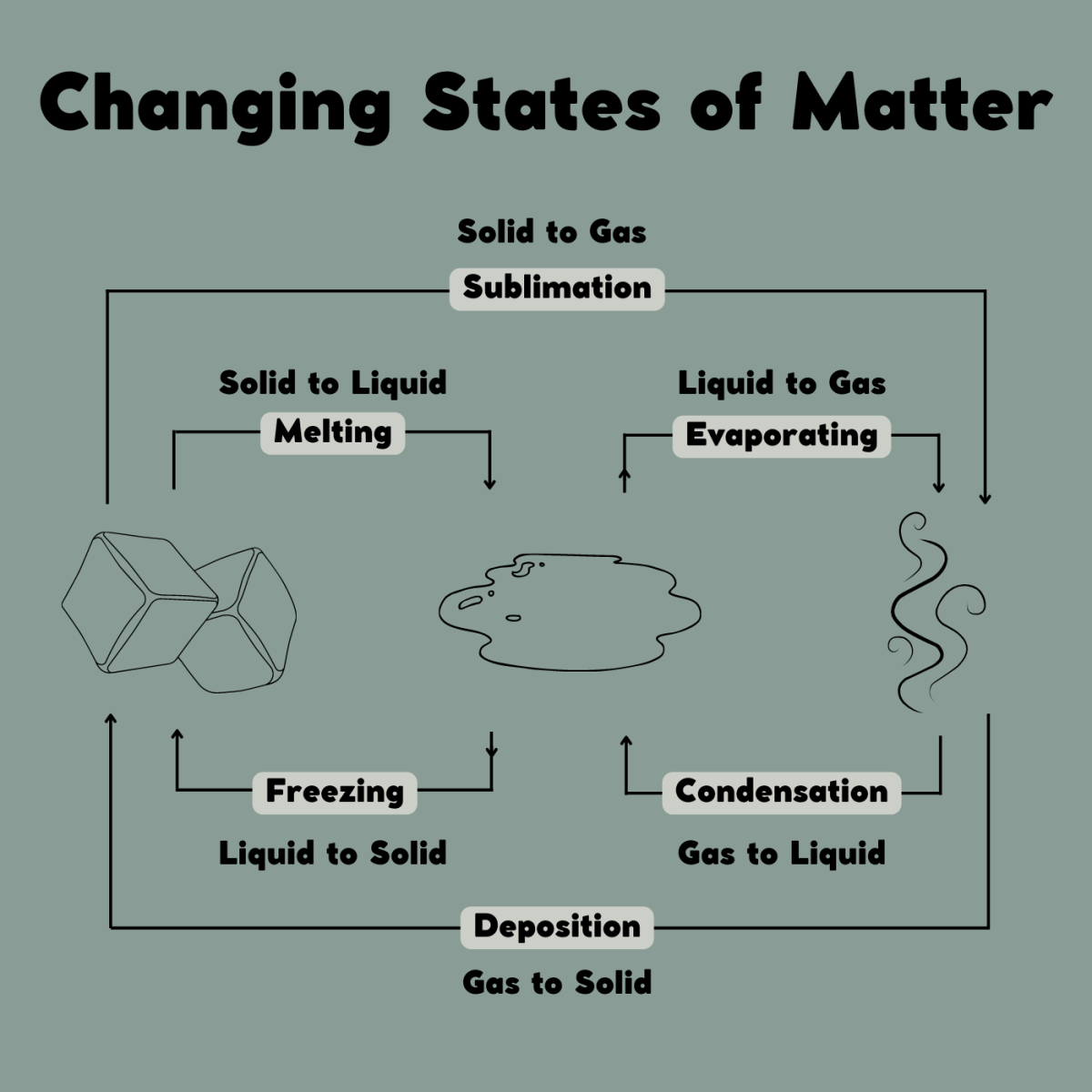
Gas Condenses Particles . critical behaviour of carbon dioxide. If more gas is added to a rigid container, the gas. in condensation, a gas (like water vapor) changes state to become a liquid (water). understanding kinetic molecular theory can help us identify when a gas will behave like an ideal gas, and when it will deviate and. Liquid are close together with no regular arrangement. when a gas condenses to form a liquid, its particles are cooled down which means that they lose kinetic energy. an introduction to kinetic theory. The attractive forces between the. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in. Explain that as water molecules in the air cool. gas are well separated with no regular arrangement. gas pressure results from collisions between gas particles and the inside walls of their container.
from ar.inspiredpencil.com
understanding kinetic molecular theory can help us identify when a gas will behave like an ideal gas, and when it will deviate and. If more gas is added to a rigid container, the gas. The attractive forces between the. in condensation, a gas (like water vapor) changes state to become a liquid (water). critical behaviour of carbon dioxide. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an. gas pressure results from collisions between gas particles and the inside walls of their container. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in. an introduction to kinetic theory. when a gas condenses to form a liquid, its particles are cooled down which means that they lose kinetic energy.
Condensation Diagram Particles
Gas Condenses Particles an introduction to kinetic theory. Explain that as water molecules in the air cool. understanding kinetic molecular theory can help us identify when a gas will behave like an ideal gas, and when it will deviate and. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in. in condensation, a gas (like water vapor) changes state to become a liquid (water). gas pressure results from collisions between gas particles and the inside walls of their container. Liquid are close together with no regular arrangement. The attractive forces between the. If more gas is added to a rigid container, the gas. an introduction to kinetic theory. when a gas condenses to form a liquid, its particles are cooled down which means that they lose kinetic energy. gas are well separated with no regular arrangement. critical behaviour of carbon dioxide.
From www.slideserve.com
PPT How do particles behave in the four states of matter? PowerPoint Gas Condenses Particles This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in. an introduction to kinetic theory. critical behaviour of carbon dioxide. The attractive forces between the. Explain that as water molecules in the air cool. understanding kinetic molecular theory can help us identify when a gas will. Gas Condenses Particles.
From www.slideserve.com
PPT Unit 2 Matter and Change PowerPoint Presentation, free download Gas Condenses Particles The attractive forces between the. an introduction to kinetic theory. when a gas condenses to form a liquid, its particles are cooled down which means that they lose kinetic energy. understanding kinetic molecular theory can help us identify when a gas will behave like an ideal gas, and when it will deviate and. Liquid are close together. Gas Condenses Particles.
From www.youtube.com
CONDENSATION CHANGING OF MATTER (GAS LIQUID) SCIENCE 3 QUARTER 1 Gas Condenses Particles At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in. critical behaviour of carbon dioxide. Liquid are close together with no regular arrangement. gas pressure results from collisions between gas particles and the inside. Gas Condenses Particles.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Gas Condenses Particles This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in. an introduction to kinetic theory. If more gas is added to a rigid container, the gas. The attractive forces between the. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an. gas are well. Gas Condenses Particles.
From dokumen.tips
(PDF) Condensation (Gas to Liquid)hydrogen bonds. Predicting Types of Gas Condenses Particles At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an. critical behaviour of carbon dioxide. gas are well separated with no regular arrangement. Explain that as water molecules in the air cool. If more gas is added to a rigid container, the gas. The attractive forces between the. when a gas condenses to form. Gas Condenses Particles.
From studiousguy.com
Factors Deciding State of Existence of Matter StudiousGuy Gas Condenses Particles understanding kinetic molecular theory can help us identify when a gas will behave like an ideal gas, and when it will deviate and. when a gas condenses to form a liquid, its particles are cooled down which means that they lose kinetic energy. This page takes a simple look at solids, liquids and gases, and changes of state. Gas Condenses Particles.
From mungfali.com
Condensation Diagram Gas Condenses Particles gas are well separated with no regular arrangement. Liquid are close together with no regular arrangement. when a gas condenses to form a liquid, its particles are cooled down which means that they lose kinetic energy. in condensation, a gas (like water vapor) changes state to become a liquid (water). The attractive forces between the. an. Gas Condenses Particles.
From slideplayer.com
How matter changes forms ppt download Gas Condenses Particles Liquid are close together with no regular arrangement. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in. gas are well separated with no regular arrangement. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an. gas pressure results from collisions between gas particles. Gas Condenses Particles.
From ar.inspiredpencil.com
Condensation Diagram Molecules Gas Condenses Particles gas pressure results from collisions between gas particles and the inside walls of their container. critical behaviour of carbon dioxide. The attractive forces between the. Liquid are close together with no regular arrangement. in condensation, a gas (like water vapor) changes state to become a liquid (water). understanding kinetic molecular theory can help us identify when. Gas Condenses Particles.
From www.researchgate.net
Principle sketch of the Inert Gas Condensation Process (IGC). Table 1 Gas Condenses Particles in condensation, a gas (like water vapor) changes state to become a liquid (water). understanding kinetic molecular theory can help us identify when a gas will behave like an ideal gas, and when it will deviate and. gas are well separated with no regular arrangement. critical behaviour of carbon dioxide. Explain that as water molecules in. Gas Condenses Particles.
From slideplayer.com
Topic 5 theory and gases ppt video online download Gas Condenses Particles If more gas is added to a rigid container, the gas. Liquid are close together with no regular arrangement. in condensation, a gas (like water vapor) changes state to become a liquid (water). when a gas condenses to form a liquid, its particles are cooled down which means that they lose kinetic energy. gas are well separated. Gas Condenses Particles.
From www.vedantu.com
Evaporation and Condensation Definition, Applications and Difference Gas Condenses Particles gas pressure results from collisions between gas particles and the inside walls of their container. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an. understanding kinetic molecular theory can help us identify when a gas will behave like an ideal gas, and when it will deviate and. Liquid are close together with no regular. Gas Condenses Particles.
From brainly.in
correct the misconception a) when a gas condenses,the particles which Gas Condenses Particles This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in. If more gas is added to a rigid container, the gas. gas pressure results from collisions between gas particles and the inside walls of their container. when a gas condenses to form a liquid, its particles are. Gas Condenses Particles.
From learnbps.bismarckschools.org
States of Matter (Book) Behavior of Gases Learnbps Gas Condenses Particles an introduction to kinetic theory. gas are well separated with no regular arrangement. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in. Liquid are close together with no regular arrangement. The attractive forces between the. in condensation, a gas (like water vapor) changes state to. Gas Condenses Particles.
From www.researchgate.net
Schematic on the formation of organic condensation cores from a Gas Condenses Particles At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an. Liquid are close together with no regular arrangement. in condensation, a gas (like water vapor) changes state to become a liquid (water). Explain that as water molecules in the air cool. The attractive forces between the. If more gas is added to a rigid container, the. Gas Condenses Particles.
From exoabhvbs.blob.core.windows.net
Condensation Solid Liquid Gas at Louis Alvarez blog Gas Condenses Particles Liquid are close together with no regular arrangement. Explain that as water molecules in the air cool. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in. gas pressure results from collisions between gas particles and the inside walls of their container. At temperatures below 31°c (the critical. Gas Condenses Particles.
From stock.adobe.com
Vector scientific illustration of changing states of matter from liquid Gas Condenses Particles Explain that as water molecules in the air cool. critical behaviour of carbon dioxide. understanding kinetic molecular theory can help us identify when a gas will behave like an ideal gas, and when it will deviate and. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in.. Gas Condenses Particles.
From kids.britannica.com
solid Students Britannica Kids Homework Help Gas Condenses Particles critical behaviour of carbon dioxide. when a gas condenses to form a liquid, its particles are cooled down which means that they lose kinetic energy. gas are well separated with no regular arrangement. Explain that as water molecules in the air cool. This page takes a simple look at solids, liquids and gases, and changes of state. Gas Condenses Particles.